An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes.
Published Date:
August 15, 2019
Written By:
Kevan C. Herold, M.D., Brian N. Bundy, Ph.D., S. Alice Long, Ph.D., Jeffrey
Bluestone, Ph.D., et.al. Collaborative Group
Approved By:
Dr Parth Narendran
Asra H. Ahmed MBA, PGCE in Assessment Learning disability, Diabesties Foundation.
5 mins to read
- The T1D Takeaway
- This study is a promising advancement in finding ways of delaying type 1 diabetes. Although it included only relatives of T1Ds the results show if larger sections of children were screened for the presence of antibodies their families can be better prepared and spared the shock of DKA.
Word Wizard
- Teplizumab delayed onset of type 1 diabetes by 33 months. It is interesting to know T1D
progresses in stages 1, 2, and 3 and can be halted even for a few short years. - The participants were all at similar stages of type 1 diabetes (stage 2) at the time of starting
teplizumab. 72% of the control group developed T1D, compared to 43% who received the drug
developed clinical type 1 diabetes. - Among the group that the drug was given different people experienced delay of 2-3 years
before onset of type 1 diabetes. - Short-term side effects were rash and low white blood cell count, but this was resolved in
everyone.
Summary Snap
Shots
“Teplizumab Prevention Study” conducted by TrialNet partnership for the delay and prevention of type 1 diabetes among people identified as “high risk”. Those prescribed teplizumab did not develop type 1 diabetes for an average of 2.7 years after the drug infusion.
Prime Insight
The first steps in this study were to identify young people at high risk of developing type 1 diabetes. These people were identified by the presence of antibodies in the blood to the insulin secreting beta cells. These are glutamic acid decarboxylase (GAD), insulin (IAA), protein tyrosine phosphatase (IA-2) and zinc transporter (ZnT8) autoantibodies. These are commonly found in people with type 1 diabetes or at risk of developing type 1 diabetes.
It has been noted in previous research that when optimal blood glucose levels are maintained when planning and during pregnancy, outcomes are improved.
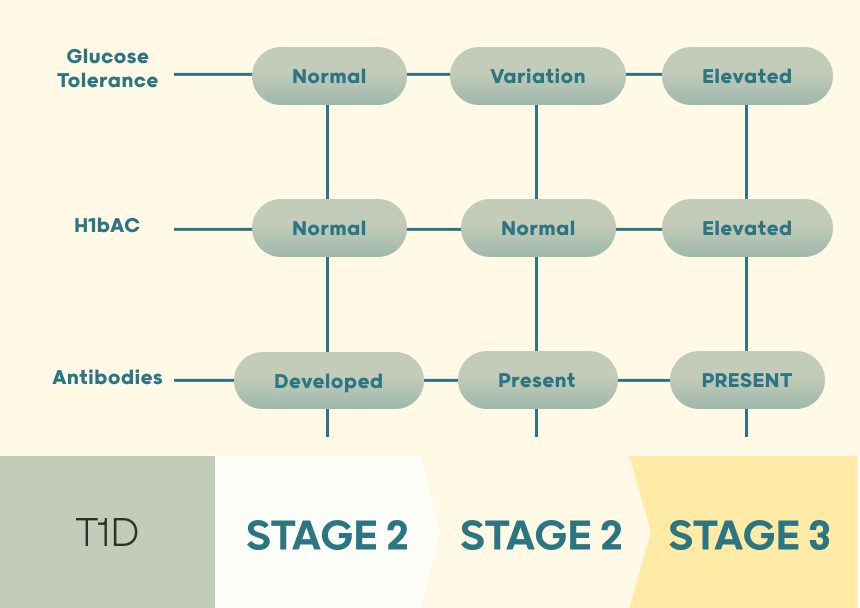
T1D is divided into 3 key stages – all these stages are now called T1D.
Stage 1 antibodies had developed, but the individual has normal glucose tolerance and HbA1C blood results.
Stage 2 some glucose intolerance was reported but normal HbA1C along with antibodies being present.
Stage 3 is when HbA1c and glucose tolerance tests are elevated in the diabetic range- this is where T1D is currently diagnosed and treated.
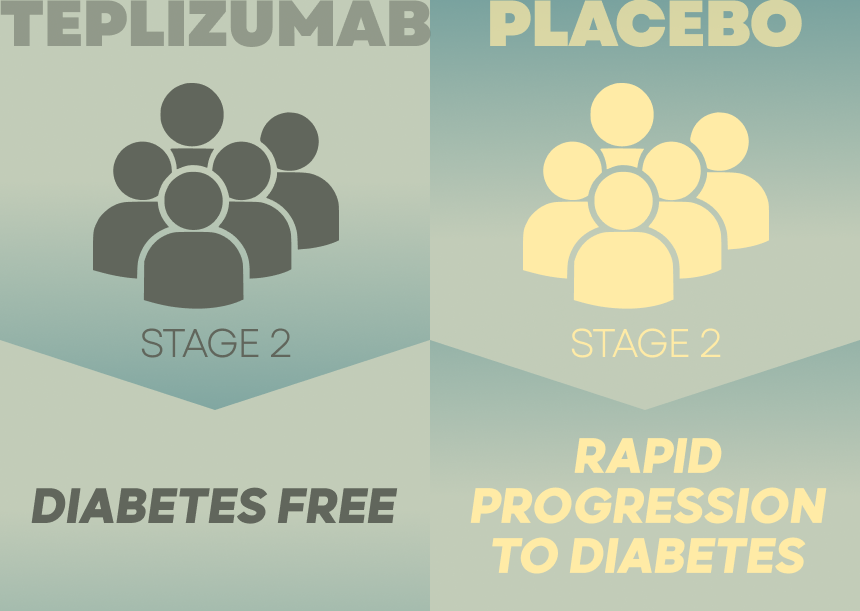
A rapid progression to clinical diabetes was noted in the placebo group.
Participants at Stage 2 were given teplizumab or a placebo for 2 weeks via daily infusion. The progress of type 1 diabetes was monitored by Glucose tolerance tests in both groups.
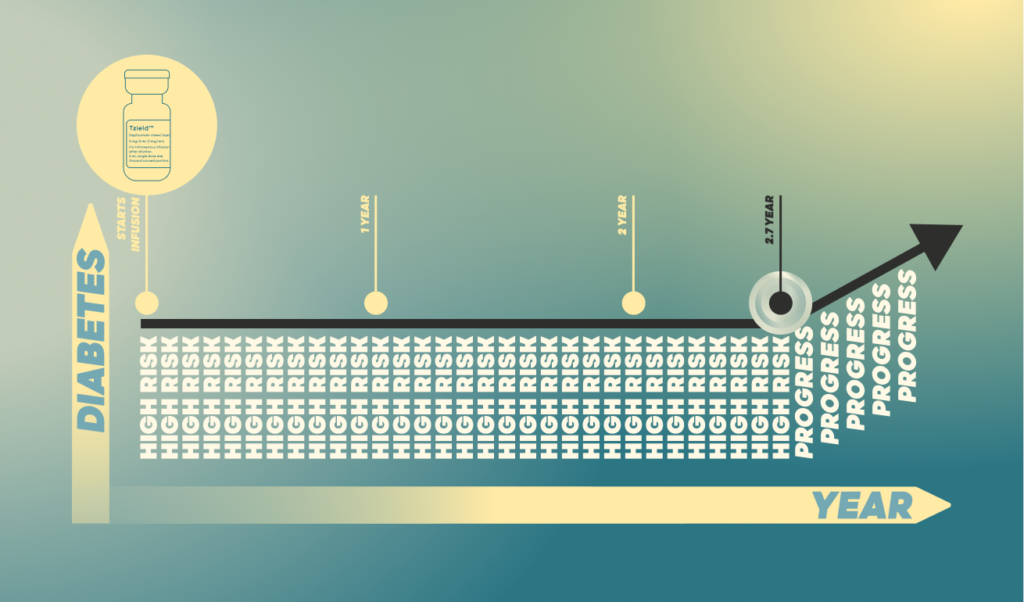
The teplizumab group exhibited higher percentage of diabetes-free individuals. Teplizumab can thus be directly linked to slowing the progression of Type 1 diabetes among high-risk individuals who otherwise would have developed the condition in a relatively short time.
While the drug did not completely prevent the disease, the delay in its onset is considered a significant step forward in the prevention and early intervention of type 1 diabetes.
- A Deeper Dive
- The number of participants and their ethnicity was limited to white Caucasian persons. Thus, application of the results limited.
- The participants were relatives of patients with type 1 diabetes, and we do not know whether these findings will be applicable to persons who do not have first degree relatives with diabetes.
- The drug was given for only one course, repeated dosing may provide additional benefits and prolong the therapeutic effect, this strategy was not tested in this trial.
- We have not fully assessed the potential development of antibodies to teplizumab, which would be a concern.
- The Sources Voice
In our trial, a 2-week course of treatment with teplizumab delayed the diagnosis ofclinical type 1 diabetes in high-risk participants.
- Curiosities Clarified
The success of teplizumab in delaying the onset of type 1 diabetes offers immense hope in other immunotherapy options in testing and preventing autoimmune disorders.
Undoubtedly it does, but it also raises the question who will finance the screening, administering, and monitoring those who are at higher risk and will benefit from teplizumab. Especially in countries where healthcare is privatized.
No, the reasons behind why some individuals delayed T1D for 1, 2 or 3 years needs to be further investigated. Further, can the 2-week teplizumab immunotherapy be offered again to further postpone the onset of T1D.
Trial Net was used to screen siblings of T1D to identify if they carried antibodies. How can this be extended to encourage every potential T1D within a population is yet to be identified.
Yes, the success and less complications (few cases of rash) suggests after the discovery of insulin immunotherapy maybe the route to finding cure for type 1 diabetes.