Efficacy and Safety of Insulin Aspart Biosimilar SAR341402 Versus Originator Insulin Aspart in People with Diabetes Treated for 26 Weeks with Multiple Daily Injections in Combination with Insulin Glargine: A Randomized Open-Label Trial (GEMELLI 1)
Published Date:
22nd Jan 2020
Published By:
Satish K. Garg, MD, Karin Wernicke-Panten, MD, Marek Wardecki, MD, Daniel Kramer, PhD, Francois Delalande, MSc, Edward Franek, MD, Karita Sadeharju, MD, Travis Monchamp, MD, Bhaswati Mukherjee, MD, and Viral N. Shah, MD
Approved By:
Dr Viral Shah
MD, Professor of Medicine, Division of Endocrinology and Metabolism
Decoded By:
Asra H. Ahmed MBA, PGCE in Assessment Learning disability, Diabesties Foundation
10 mins to read
- The T1D Takeaway
- Diabetes management just got a makeover with biosimilar insulin. It’s like our pancreas hired a stunt double—same action, but at a fraction of the cost. Now even our insulin has a twin!
Word Wizard
- The GEMELLI 1 study compared the efficacy and safety of the biosimilar insulin aspart (SAR341402) to the original insulin aspart (NovoLog/NovoRapid) in people with diabetes over 26 weeks.
- Efficacy: Both insulins demonstrated similar efficacy in terms of glycemic control.
- Safety: The safety profiles of SAR341402 and the original insulin were comparable, with no significant differences in adverse events.
- Immune response: Both treatments had similar immune response profiles, indicating no increased risk of immune reactions with the biosimilar.

Summary Snap
Shots
- This study was conducted over a period of 26 weeks (6 months) among 597 individuals living with either type 1 or type 2 diabetes, but on multiple daily injections. These individuals received both insulins showed similar improvements in HbA1c levels to Novo Rapid Aspart and displayed comparable safety profiles, including hypoglycemia and adverse events.
- SAR-Asp or NN-Asp was used alongside insulin glargine. No adverse immune response was recorded in both groups.
Prime Insight
Insulin Aspart is a fast-acting insulin used for Type 1 and Type 2 Diabetes. In this study, a thorough approach was taken to ensure both efficacy and safety during the use by those participating in it.
These results support the use of SAR341402 as a viable alternative to the originator insulin aspart for managing diabetes with multiple daily injections combined with insulin glargine.

Medications were provided on day 1 until the end of the study via 3ml prefilled pens to one group and Novorapid flex pens apart to the second group. Both were of 100L/mL subcutaneous injection pre meal. Adherence to treatment was monitored by tracking and revieing diaries and counting the number of insulin pens used.
The starting dose was a unit-to-unit conversion from prior insulin use, adjusted based on self-monitored plasma glucose (SMPG) and meal carbohydrate content. Post-meal glucose levels were targeted below 180 mg/dL and pre-meal levels between 80-130 mg/dL.
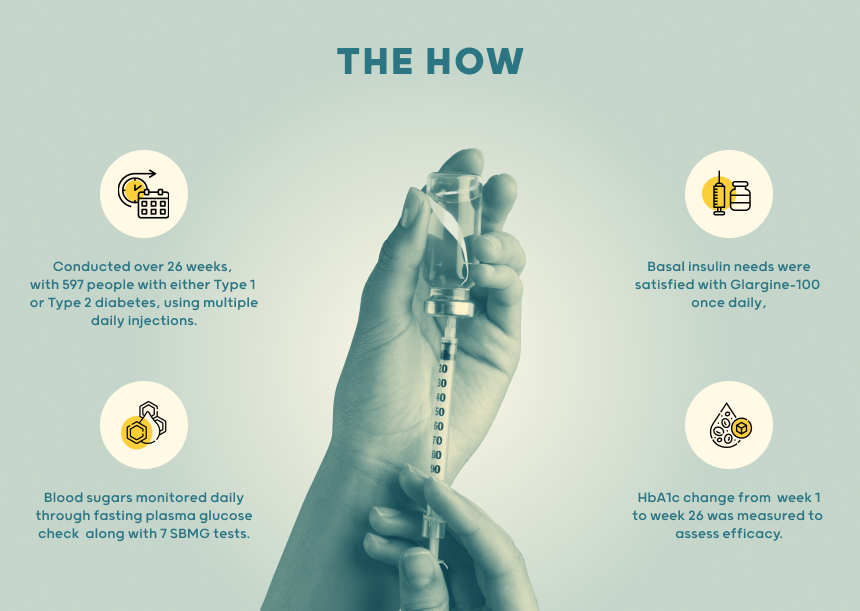
Basal insulin needs were satisfied with Glargine-100 once daily, injected at a consistent time to maintain stable blood sugar levels.
Blood sugars were monitored through fasting plasma glucose check along with seven SBMG tests over 24 hours. Further HbA1c was measured.
Primary Efficacy Endpoint: Focused on the change in HbA1c from baseline to week 26 to assess treatment effectiveness. For Safety assessment hypoglycemia events and antibody response was used. These showed that SAR-Aspart is a well-tolerated, effective, and safe alternative to NovoRapid Aspart insulin for treating diabetes.
Initially, both groups showed similar percentage of positive antibodies (AIAs) and over the six-month treatment period, the development of new or boosted AIAs was comparable between groups. AIA levels remained stable throughout the study.
The standout conclusion of the GEMELLI 1 study is that the Insulin Aspart biosimilar SAR341402 is not inferior to the original insulin Aspart (NN-Asp) in terms of efficacy and safety for controlling blood glucose levels in people with diabetes.
- A Deeper Dive
- The Sources Voice
“In summary, the findings of this study suggest that SAR-Asp is a well-tolerated, effective, and safe treatment option when prescribed for participants with diabetes who have had prior treatment with other commercial mealtime insulin analog therapies.”
- Curiosities Clarified
Yes, SAR341402, the biosimilar insulin aspart, provides the same level of glycemic control as the original insulin aspart. The GEMELLI 1 study found that participants who switched to SAR341402 achieved similar efficacy and safety outcomes compared to those who continued their pre-study insulin aspart treatment.
Yes, SAR341402 (Biosimilar insulin) is as safe as Novorapid, particularly regarding adverse events and hypoglycemia rates. The safety profiles of SAR341402 and Novorapid were comparable, with similar incidences of adverse events, including hypoglycemia.
The findings at the end of the study strongly indicated SAR341402 biosimilar insulin is comparable to the original NoVo rapid in terms of efficacy, safety, and its immune response, supporting its use as a biosimilar for diabetes management.