Stem cells differentiation into insulin-producing cells (IPCs): recent advances and current challenges
Published Date:
15 July 2022.
Published By:
Silva IBB, Kimura CH, Colantoni VP, Sogayar MC.
Approved By:
James Shaw
Decoded By:
Asra H. Ahmed
MBA, PGCE in Assessment Learning disability, Diabesties Foundation.
10 mins to read
- The T1D Takeaway
- This review of different studies raises the bar for those living with type 1 diabetes that a mini-invasive placement of differentiated calls can replace insulin producing beta cells. Where our own body will respond to a meal stimulus to produce insulin.
- Current organ transplants require immunosuppressants. Many of those living with type 1 diabetes who have had pancreas transplants are now living free of insulin therapy. However, they need drugs to maintain the viability of the transplanted organ. With new multidisciplinary methods it is hoped that such drugs and their side effects become a thing of the past.
- To live free of injections and to just taste the food without doing a mental carbohydrate calculation foreseeing a hypoglycemia is what dreams are made of for anyone who is intensively managing their condition. Indeed, stem cells are now being differentiated into new cell clusters ‘islets’ which make both insulin and glucagon in a fully regulated way.
Word Wizard
- The International Diabetes Federation predicts that by 2045, 700 million adults worldwide will have diabetes. Furthermore, there is a need for finding better therapies for children and teenagers under 20 who live with Type 1 Diabetes (T1D) to avoid long term complications.
- Insulin therapy is the primary treatment, but it requires intensive continuous management and monitoring with limited effectiveness and monitoring by the person living with diabetes. This requires daily hard work without a break and can never completely normalize blood glucose.

Summary Snap
Shots
In 2014, breakthrough research achieved a protocol for making insulin-producing cells resembling human pancreatic beta cells from stem cells. These cells made insulin in relatively high amounts and released this immediately in response to high glucose levels. The current Vertex clinical trial therapy uses these cells which were originally created by ground-breaking research undertaken by Dr Doug Melton in his labs at Harvard.
Prime Insight
Different cell therapies are:
Immunotherapy: Focuses on manipulating the immune system’s cells to target and eliminate cancer cells or cells causing autoimmune diseases.
Cell Transplantation: Involves the direct transplantation of cells, such as pancreatic islet cells for diabetes treatment or bone marrow stem cells for certain blood disorders.
Gene-Modified Cell Therapy: Involves modifying cells taken from an individual or another donor and introducing or altering genes to correct genetic defects or drive new functions. These modified cells are then implanted into the recipient to have their therapeutic effects. The focus here is on beta cell transplantation through generation of insulin-producing tissue for cell therapy.
If cells come from someone else, immunosuppressant drugs are required to stop them being recognized and attacked by the immune system.
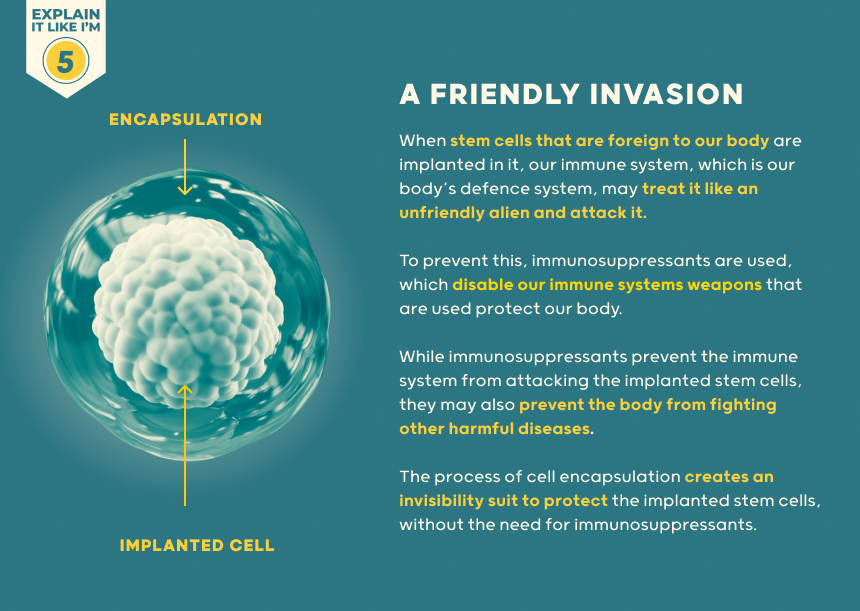
If they look enough like a normal beta-cell, they can be attacked in someone with type 1 diabetes by their autoimmune process - even if they are their own cells. Immunosuppression has potentially serious side effects by damping down the immune system.
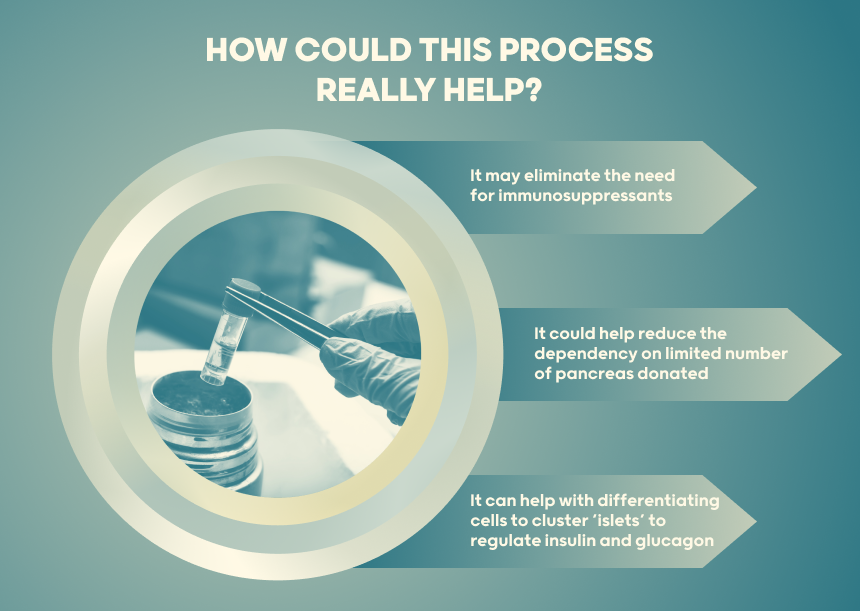
This increases risk of skin and other cancers as well as infection. To overcome this, surrounding the cells with capsules to shield them from the immune system is being explored. There is a limit to the number of donors who have generously donated their pancreas after death for extraction of insulin-producing cells. Using stem cells offers the potential of generating enough cells for everyone with diabetes.
- Current insulin therapy doesn’t replicate the body’s natural insulin release well, leading to challenges in preventing harmful effects of high/low blood sugars.
- Inhalable insulin, and automated pumps cannot yet prevent high or very low blood sugars. Transplantation of insulin-producing tissue, like pancreatic islets, may offer a promising option for restoring pancreatic cell function, enhance quality of life and reduce the burden of having to make multiple decisions every day to manage diabetes.
- In this research, methods have been developed to grow insulin making cells outside the body, using strict rules and checks to make sure they are safe for people.
- Two companies, ViaCyte and Vertex, are involved in trials using stem cells. These have shown very promising outcomes.
- There are different ways to get insulin-producing cells (IPCs). Some ways involve letting cells grow on their own or using specific factors to guide their pathway of differentiation. Current trials are using this approach. Alternatively new genes which drive differentiation or make the cells more invisible to the immune system can be inserted into the cells for gene-modified cell therapy.
- Challenges remain, but progress has been made thanks to collaborative across different fields of study. Efforts in stem cell biology, embryology, immunology, cell encapsulation, and tissue bioengineering aim to create effective cellular therapies.
- The next major goal is within site: Obtain mature cells with high glucose-stimulated insulin secretion capacity enabling a single transplant to produce enough insulin to take away the need for insulin injections or pumps. This currently still requires immunosuppression while trials are underway to test whether transplanted cells survive effectively inside protective capsules.
The generation of pancreatic β-cells from stem cells holds significant promise as a therapeutic approach to achieve insulin independence for millions of persons living with Diabetes.
Ongoing efforts are focused on optimizing differentiation, encapsulation techniques and bioengineering to produce sustainable long-term cells that can maintain glycemic control.
- A Deeper Dive
- Further research in trans differentiation.
- Vertex and Viacite study in stem cell differentiation. Where are we at and what are the next steps?
- The Sources Voice
Here, we provide an overview of the current approaches and achievements in obtaining stem cell-derived β-cells and the numerous challenges, which still need to be overcome to achieve the ultimate goal.
Clinical translation of stem cells-derived β-cells for efficient maintenance of long-term normal glucose remains an unmet need. Nevertheless, progress is being made on all fronts towards production of functional and mature β-cells and integration of interdisciplinary fields to generate efficient cell therapy strategies capable of reversing the clinical outcome of T1D.
- Curiosities Clarified
The review has highlighted both ViaCyte and Vertex protocols as successful demonstration of using stem cell differentiation to produce useful amounts of insulin for sustained glycemic control and less hypoglycemic episodes.
It does appear that encapsulation can prevent immune attack including type 1 diabetes autoimmunity. Whether capsules can be engineered to provide a good blood supply without breaching the ‘tea bag’ semi-permeable barrier between the cells and the immune system – so that cells do not die from oxygen-starvation, remains to be seen. Although cell therapy is not yet ready for everyone, this is a fast-moving and exciting time with trials available already to pioneering research volunteers with type 1 diabetes.
3 Comments
For all your sports betting needs, ‘Codere Deportes’ has you covered. Great odds and a huge selection of sports. I’m always finding something to bet on! codere deportes
Ready to win big! Considering my options on apostamax bet. Any advice from experienced players welcome!
I was searching for tai awin68 download and I got redirected to their official website, it was that easy. Check it out here: tai awin68