Vertex Presents Positive VX-880 Results From Ongoing Phase 1/2 Study in Type 1 Diabetes.
Published Date:
October 3, 2023, an update on clinical research.
Published By:
Vertex Pharmaceuticals
Approved By:
Prof. Pratik Choudhary
Decoded By:
Dr Kunjal Jain, BHMS ,PGDHHM & Asra H. Ahmed MBA, PGCE in Assessment Learning disability, Diabesties Foundation
10 mins to read
- The T1D Takeaway
- Recent news has cast a doubt on the efficacy of VX-880 treatment using lab grown stem cells that produced insulin. Vertex Pharmaceticules released a statement announcing the deaths of two tria participants. The study is currently paused.
- However, upon closer look they announced the deaths were not related to the research product. Both candidates lived with other conditions and experienced severe hypoglycemia. They were using immuno-suppresants which may have influenced their unexpected demise. As always we practice caution when celebrating new breakthrough or set backs related to type 1 diabetes management or cure.
- These findings mark a significant advancement in the treatment of Type1 Diabetes, moving closer to a possible cure.
- After one year into VX-880 therapy, the HbA1c returned to normal without the need for exogenous insulin, a remarkable development that raises hopes for the efficaciousness of these transformative therapies.
Word Wizard
- Prior to treatment all six enrolled persons had long-standing type 1 diabetes with no internal insulin secretion.
- On Day 90, the trial showed that transplanted islet cells became part of the body and produced insulin in response to glucose during a test where a mix of carbohydrate, protein and fats was given and then the body’s response in blood sugar variability was monitored.
- All individual showed improved blood sugar levels, decreased Hba1c and improved time in range when continuous glucose and insulin production was closely monitored.
- They showed marked reduction or complete elimination of externally administered insulin.

Summary Snap
Shots
“Maintaining blood glucose levels as close to non-diabetic range as safely as possible reduces the occurrence of long term T1D- related complications, and people can more likely achieve lower blood glucose levels with the help of intensive insulin therapy.”
Prime Insight
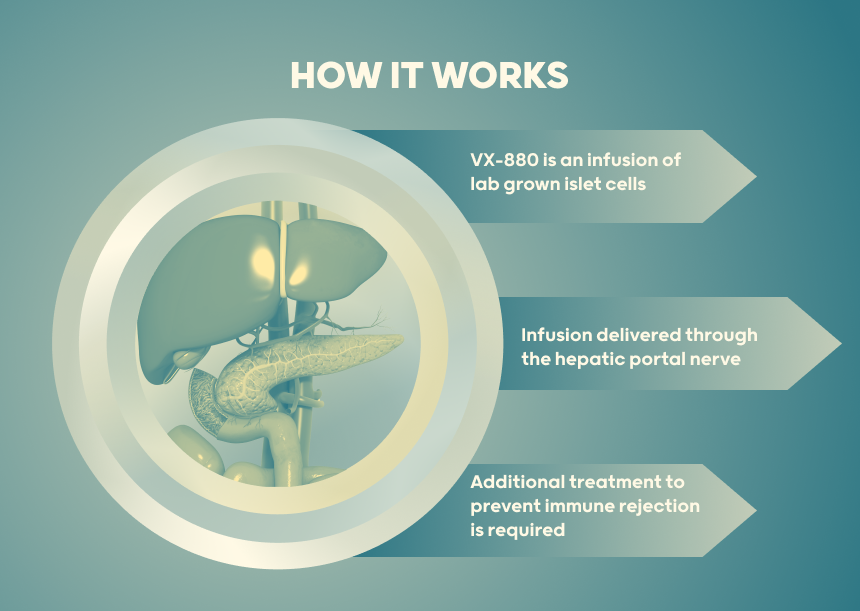
Individuals living with Type 1 diabetes (T1D) having reduced hypoglycemic awareness and severe hypoglycemic events (SHEs) were chosen as study cohort. VX-880 is delivered by an infusion into the hepatic portal vein, (blood vessel that connects the digestive organs to the liver). It requires chronic immunosuppressive therapy, that is additional treatment designed to suppress the activity of the immune system. The immune system plays a crucial role in defending the body against foreign invaders, such as bacteria, viruses, and abnormal cells to protect the islet cells from immune rejection.
Baseline Characteristics of the six persons in the clinical trial:
- All six persons living with T1D showed no evidence of own insulin secretion (undetectable fasting C-peptide).
- History of recurrent severe hypoglycemic episode were recorded in the year prior to treatment.
- Required an average of 34.0 units of insulin per day before VX-880 therapy.
VX-880 is delivered by an infusion into the hepatic portal vein, (blood vessel that connects the digestive organs to the liver)
The development of novel medical therapies is an ongoing process, and the status of VX-880 is still under evaluation.
Treatment Administered: VX-880, is an experimental stem cell-derived islet cell therapy designed for the treatment of type 1 diabetes (T1D). It’s important to note that the development of novel medical therapies is an ongoing process, and the status of VX-880 is still under evaluation. This is an update reported at the American Diabetes Association June 2023 and October 2023.
Post-Treatment Outcomes:
- All six individuals showed post-treatment own insulin secretion.
- Improved glycemic control (measured by HbA1c).
- Enhanced time-in-range on continuous glucose monitoring.
- Reduced or eliminated the need for external insulin.
Follow-up Period:
- Persons with over 90 days of follow-up had no SHEs during the evaluation period.
- One person living with type 1 diabetes in Part A received a half-target dose and was followed for approximately nine months.
- Two individuals in Part B, followed for at least 12 months, met the primary endpoint, becoming insulin independent, i.e. were able to produce insulin from within.
- In part B of the trial, three more individuals received the full VX-880 dose. Their follow-up between 29 and 90 days showed improved insulin secretion, reduced HbA1c, better glucose time-in-range, and decreased daily insulin use.
- Demonstrated that the inserted cells became part of the body and made insulin when sugar was present during the Day 90 food test.

Safety and Tolerance:
VX-880 was well-tolerated; most adverse events were mild or moderate. Common adverse events included dehydration, diarrhea, hypomagnesemia, and rash. No serious adverse events related to VX-880 treatment were reported.
Committee Recommendation:
The independent data review committee recommended advancing to Part C of the trial based on positive safety and efficacy outcomes in Parts A and B.
After treatment, all six persons living with type 1 diabetes showed improved glycemic control, internal insulin production, and reduced or eliminated insulin taken from outside.
- A Deeper Dive
- The Sources Voice
“We continue to marvel at the impressive data from the VX-880 program as evidenced by the improvements in all treated patients across all glycemic measures,” said Trevor Reichman, M.D., Department of Surgery, University of Toronto. “This represents an incredibly promising investigational therapy, one with far-reaching potential.”
- Curiosities Clarified
With this study medical science is getting closer to cure of type 1 as in this study still Part C study and other steps are remaining to conduct.
There were no major adverse events associated with VX-880 therapy, and the bulk of AEs were mild or moderate. Dehydration, diarrhea, hypomagnesaemia, and rash were the most frequent adverse events. One subject experienced SHEs during the perioperative phase, as was previously reported.